The starting materials are:
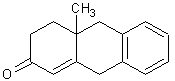
The product of this reaction will form a six-membered ring and three new carbon-carbon bonds. First an enolate will form from the first starting material. Then nucleophilic attack of the beta carbon and protonation occurs. Next, an intramolecular aldol reaction will take place to form a beta-hydroxy ketone. Lastly, the material will be dehyrated to form the final alpha,beta-unsaturated ketone as follows.
An easy way to draw the product of this type of reaction is to place the alpha carbon of the compound that becomes the enolate next to the beta carbon of the alpha,beta-unsaturated carbonyl compound. Then, join the appropriate carbons together so that a new six-membered ring forms. To better understand the Robinson Annulation reaction, see section 24.9 starting on page 936 of our textbook. I think that a problem like this might about on our next exam because it incorporates topics that we have previously discussed and other reactions we have learned about in chapter 24.
An easy way to draw the product of this type of reaction is to place the alpha carbon of the compound that becomes the enolate next to the beta carbon of the alpha,beta-unsaturated carbonyl compound. Then, join the appropriate carbons together so that a new six-membered ring forms. To better understand the Robinson Annulation reaction, see section 24.9 starting on page 936 of our textbook. I think that a problem like this might about on our next exam because it incorporates topics that we have previously discussed and other reactions we have learned about in chapter 24.

No comments:
Post a Comment